Microbiota in disease-transmitting vectors
Haematophagous arthropods, including mosquitoes, ticks, flies, triatomine bugs and lice (here referred to as vectors), are involved in the transmission of various pathogens to mammals on whom they blood feed. The diseases caused by these pathogens, collectively known as vector-borne diseases (VBDs), threaten the health of humans and animals. Although the vector arthropods differ in life histories, feeding behaviour as well as reproductive strategies, they all harbour symbiotic microorganisms, known as microbiota, on which they depend for completing essential aspects of their biology, such as development and reproduction. In this Review, we summarize the shared and unique key features of the symbiotic associations that have been characterized in the major vector taxa. We discuss the crosstalks between microbiota and their arthropod hosts that influence vector metabolism and immune responses relevant for pathogen transmission success, known as vector competence. Finally, we highlight how current knowledge on symbiotic associations is being explored to develop non-chemical-based alternative control methods that aim to reduce vector populations, or reduce vector competence. We conclude by highlighting the remaining knowledge gaps that stand to advance basic and translational aspects of vector–microbiota interactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
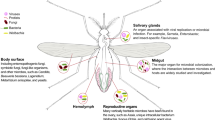
Holobiont perspectives on tripartite interactions among microbiota, mosquitoes, and pathogens
Article 25 May 2023
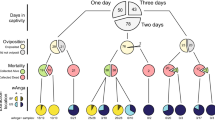
The Anopheles coluzzii microbiome and its interaction with the intracellular parasite Wolbachia
Article Open access 14 August 2020
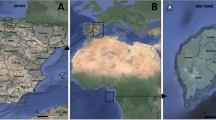
Characterization of the microbiome of Aedes albopictus populations in different habitats from Spain and São Tomé
Article Open access 04 September 2024
References
- World Health Organization. Global vector control response (2017–2030). World Health Organizationhttps://www.who.int/publications-detail-redirect/9789241512978/ (2017).
- Bogiitsh, B. J., Carter, C. E. & Oeltmann, T. N. Human Pararsitology 4th edn (Academic, 2013).
- World Health Organization. Vector-borne diseases. World Health Organizationhttps://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
- Socha, W., Kwasnik, M., Larska, M., Rola, J. & Rozek, W. Vector-borne viral diseases as a current threat for human and animal health—One Health perspective. J. Clin. Med.11, 3026 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Kurokawa, C. et al. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol.18, 587–600 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wondim, M. A. et al. Epidemiological trends of trans-boundary tick-borne encephalitis in Europe, 2000–2019. Pathogens11, 704 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Piotrowski, M. & Rymaszewska, A. Expansion of tick-borne rickettsioses in the World. Microorganisms8, 1906 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Madison-Antenucci, S., Kramer, L. D., Gebhardt, L. L. & Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev.33, e00083-18 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Michelitsch, A., Wernike, K., Klaus, C., Dobler, G. & Beer, M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses11, 669 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis.14, e0007831 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Beaty, B. J. & Marquardt, W. C. The Biology of Disease Vectors (Univ. Press of Colorado, 1996).
- Jimenez-Cortes, J. G. et al. Bacterial symbionts in human blood-feeding arthropods: Patterns, general mechanisms and effects of global ecological changes. Acta Trop.186, 69–101 (2018). ArticlePubMedGoogle Scholar
- Song, X., Zhong, Z., Gao, L., Weiss, B. L. & Wang, J. Metabolic interactions between disease-transmitting vectors and their microbiota. Trends Parasitol.38, 697–708 (2022). ArticleCASPubMedGoogle Scholar
- Shaw, W. R. & Catteruccia, F. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat. Microbiol.4, 20–34 (2019). ArticleCASPubMedGoogle Scholar
- Moran, N. A. Symbiosis. Curr. Biol.16, R866–R871 (2006). ArticleCASPubMedGoogle Scholar
- Khachane, A. N., Timmis, K. N. & Martins dos Santos, V. A. Dynamics of reductive genome evolution in mitochondria and obligate intracellular microbes. Mol. Biol. Evol.24, 449–456 (2007). ArticleCASPubMedGoogle Scholar
- Malassigne, S., Valiente Moro, C. & Luis, P. Mosquito mycobiota: an overview of non-entomopathogenic fungal interactions. Pathogens9, 564 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Altinli, M., Schnettler, E. & Sicard, M. Symbiotic interactions between mosquitoes and mosquito viruses. Front. Cell Infect. Micrbiol.11, 694020 (2021). ArticleCASGoogle Scholar
- Brito, T. F. et al. Transovarial transmission of a core virome in the Chagas disease vector Rhodnius prolixus. PLoS Pathog.17, e1009780 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kariithi, H. M. et al. Coevolution of hytrosaviruses and host immune responses. BMC Microbiol.18, 183 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gao, H., Cui, C., Wang, L., Jacobs-Lorena, M. & Wang, S. Mosquito microbiota and implications for disease control. Trends Parasitol.36, 98–111 (2020). ArticlePubMedGoogle Scholar
- Campolina, T. B., Villegas, L. E. M., Monteiro, C. C., Pimenta, P. F. P. & Secundino, N. F. C. Tripartite interactions: Leishmania, microbiota and Lutzomyia longipalpis. PLoS Negl. Trop. Dis.14, e0008666 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Wang, S. et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science357, 1399–1402 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Maffo, C. G. T. et al. Molecular detection and maternal transmission of a bacterial symbiont Asaia species in field-caught Anopheles mosquitoes from Cameroon. Parasit. Vectors14, 539 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Caragata, E. P., Dutra, H. L. C., Sucupira, P. H. F., Ferreira, A. G. A. & Moreira, L. A. Wolbachia as translational science: controlling mosquito-borne pathogens. Trends Parasitol.37, 1050–1067 (2021). ArticlePubMedGoogle Scholar
- Vasilakis, N. et al. Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. J. Virol.87, 2475–2488 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Cappelli, A., Favia, G. & Ricci, I. Wickerhamomyces anomalus in mosquitoes: a promising yeast-based tool for the “symbiotic control” of mosquito-borne diseases. Front. Microbiol.11, 621605 (2020). ArticlePubMedGoogle Scholar
- Patterson, E. I., Villinger, J., Muthoni, J. N., Dobel-Ober, L. & Hughes, G. L. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr. Opin. Insect Sci.39, 50–56 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Duron, O. & Gottlieb, Y. Convergence of nutritional symbioses in obligate blood feeders. Trends Parasitol.36, 816–825 (2020). ArticleCASPubMedGoogle Scholar
- Attardo, G. M., Scolari, F. & Malacrida, A. Bacterial symbionts of tsetse flies: relationships and functional interactions between tsetse flies and their symbionts. Results Probl. Cell Differ.69, 497–536 (2020). ArticleCASPubMedGoogle Scholar
- Pais, R., Lohs, C., Wu, Y., Wang, J. & Aksoy, S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microbiol.74, 5965–5974 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sasaki-Fukatsu, K. et al. Symbiotic bacteria associated with stomach discs of human lice. Appl. Environ. Microbiol.72, 7349–7352 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hosokawa, T., Koga, R., Kikuchi, Y., Meng, X. Y. & Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA107, 769–774 (2010). ArticleCASPubMedGoogle Scholar
- Stavru, F., Riemer, J., Jex, A. & Sassera, D. When bacteria meet mitochondria: the strange case of the tick symbiont Midichloria mitochondrii. Cell Microbiol.22, e13189 (2020). ArticleCASPubMedGoogle Scholar
- Salcedo-Porras, N., Umana-Diaz, C., Bitencourt, R. O. B. & Lowenberger, C. The role of bacterial symbionts in triatomines: an evolutionary perspective. Microorganisms8, 1438 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kariithi, H. M., Meki, I. K., Boucias, D. G. & Abd-Alla, A. M. Hytrosaviruses: current status and perspective. Curr. Opin. Insect Sci.22, 71–78 (2017). ArticlePubMedGoogle Scholar
- Neville, C. A. The Biology of Mosquitoes: Development, Nutrition and Reproduction (Chapman & Hall, 1992).
- Coon, K. L., Vogel, K. J., Brown, M. R. & Strand, M. R. Mosquitoes rely on their gut microbiota for development. Mol. Ecol.23, 2727–2739 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chouaia, B. et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol.12, S2 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, X. et al. Bacterial microbiota assemblage in Aedes albopictus mosquitoes and its impacts on larval development. Mol. Ecol.27, 2972–2985 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Vogel, K. J., Valzania, L., Coon, K. L., Brown, M. R. & Strand, M. R. Transcriptome sequencing reveals large-scale changes in axenic Aedes aegypti larvae. PLoS Negl. Trop. Dis.11, e0005273 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Coon, K. L. et al. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl Acad. Sci. USA114, E5362–E5369 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Romoli, O., Schonbeck, J. C., Hapfelmeier, S. & Gendrin, M. Production of germ-free mosquitoes via transient colonisation allows stage-specific investigation of host–microbiota interactions. Nat. Commun.12, 942 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, Y. et al. Riboflavin instability is a key factor underlying the requirement of a gut microbiota for mosquito development. Proc. Natl Acad. Sci. USA118, e2101080118 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Peterkova-Koci, K., Robles-Murguia, M., Ramalho-Ortigao, M. & Zurek, L. Significance of bacteria in oviposition and larval development of the sand fly Lutzomyia longipalpis. Parasit. Vectors5, 145 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Steyn, A., Roets, F. & Botha, A. Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb. Ecol.71, 747–760 (2016). ArticleCASPubMedGoogle Scholar
- Harington, J. S. Studies on Rhodnius prolixus: growth and development of normal and sterile bugs, and the symbiotic relationship. Parasitology50, 279–286 (1960). ArticleCASPubMedGoogle Scholar
- Guizzo, M. G. et al. A Coxiella mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep.7, 17554 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Ben-Yosef, M. et al. Coxiella-like endosymbiont of Rhipicephalus sanguineus is required for physiological processes during ontogeny. Front. Microbiol.11, 493 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Guizzo, M. G. et al. Coxiella endosymbiont of Rhipicephalus microplus modulates tick physiology with a major impact in blood feeding capacity. Front. Microbiol.13, 868575 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Zhong, Z. et al. Symbiont-regulated serotonin biosynthesis modulates tick feeding activity. Cell Host Microbe29, 1545–1557 (2021). ArticleCASPubMedGoogle Scholar
- Li, L. H., Zhang, Y. & Zhu, D. Effects of antibiotic treatment on the fecundity of Rhipicephalus haemaphysaloides ticks. Parasit. Vectors11, 242 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Zhang, C. M. et al. Endosymbiont CLS-HI plays a role in reproduction and development of Haemaphysalis longicornis. Exp. Appl. Acarol.73, 429–438 (2017). ArticlePubMedGoogle Scholar
- Zhong, J., Jasinskas, A. & Barbour, A. G. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE2, e405 (2007). ArticlePubMedPubMed CentralGoogle Scholar
- Kurlovs, A. H., Li, J., Cheng, D. & Zhong, J. Ixodes pacificus ticks maintain embryogenesis and egg hatching after antibiotic treatment of Rickettsia endosymbiont. PLoS ONE9, e104815 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Scolari, F. et al. Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans. BMC Microbiol.18, 169 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Engl, T. et al. Effect of antibiotic treatment and gamma-irradiation on cuticular hydrocarbon profiles and mate choice in tsetse flies (Glossina m. morsitans). BMC Microbiol.18, 145 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Liu, N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol.60, 537–559 (2015). ArticleCASPubMedGoogle Scholar
- Mougabure-Cueto, G. & Picollo, M. I. Insecticide resistance in vector Chagas disease: evolution, mechanisms and management. Acta Trop.149, 70–85 (2015). ArticleCASPubMedGoogle Scholar
- Coles, T. B. & Dryden, M. W. Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasit. Vectors7, 8 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Soltani, A., Vatandoost, H., Oshaghi, M. A., Enayati, A. A. & Chavshin, A. R. The role of midgut symbiotic bacteria in resistance of Anopheles stephensi (Diptera: Culicidae) to organophosphate insecticides. Pathog. Glob. Health111, 289–296 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Soh, L. S. & Veera Singham, G. Bacterial symbionts influence host susceptibility to fenitrothion and imidacloprid in the obligate hematophagous bed bug, Cimex hemipterus. Sci. Rep.12, 4919 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Muturi, E. J., Dunlap, C., Smartt, C. T. & Shin, D. Resistance to permethrin alters the gut microbiota of Aedes aegypti. Sci. Rep.11, 14406 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pelloquin, B. et al. Overabundance of Asaia and Serratia bacteria is associated with deltamethrin insecticide susceptibility in Anopheles coluzzii from Agboville, Cote d’Ivoire. Microbiol. Spectr.9, e00157-21 (2021). ArticlePubMedPubMed CentralGoogle Scholar
- Omoke, D. et al. Western Kenyan Anopheles gambiae showing intense permethrin resistance harbour distinct microbiota. Malar. J.20, 77 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Comandatore, F. et al. Phylogenomics reveals that Asaia symbionts from insects underwent convergent genome reduction, preserving an insecticide-degrading gene. mBio12, e00106–e00121 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sterkel, M., Oliveira, J. H. M., Bottino-Rojas, V., Paiva-Silva, G. O. & Oliveira, P. L. The dose makes the poison: nutritional overload determines the life traits of blood-feeding arthropods. Trends Parasitol.33, 633–644 (2017). ArticlePubMedGoogle Scholar
- Akman, L. et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet.32, 402–407 (2002). ArticleCASPubMedGoogle Scholar
- Smith, T. A., Driscoll, T., Gillespie, J. J. & Raghavan, R. A Coxiella-like endosymbiont is a potential vitamin source for the lone star tick. Genome Biol. Evol.7, 831–838 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hunter, D. J. et al. The Rickettsia endosymbiont of Ixodes pacificus contains all the genes of de novo folate biosynthesis. PLoS ONE10, e0144552 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Duron, O. et al. Tick–bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol.28, 1896–1902 (2018). ArticleCASPubMedGoogle Scholar
- Sassera, D. et al. Phylogenomic evidence for the presence of a flagellum and cbb3 oxidase in the free-living mitochondrial ancestor. Mol. Biol. Evol.28, 3285–3296 (2011). ArticleCASPubMedGoogle Scholar
- Nikoh, N. et al. Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl Acad. Sci. USA111, 10257–10262 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Boyd, B. M. et al. Primates, lice and bacteria: speciation and genome evolution in the symbionts of hominid lice. Mol. Biol. Evol.34, 1743–1757 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Belda, E., Moya, A., Bentley, S. & Silva, F. J. Mobile genetic element proliferation and gene inactivation impact over the genome structure and metabolic capabilities of Sodalis glossinidius, the secondary endosymbiont of tsetse flies. BMC Genomics11, 449 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Buysse, M. et al. A dual endosymbiosis supports nutritional adaptation to hematophagy in the invasive tick Hyalomma marginatum. eLife10, e72747 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Binetruy, F. et al. Microbial community structure reveals instability of nutritional symbiosis during the evolutionary radiation of Amblyomma ticks. Mol. Ecol.29, 1016–1029 (2020). ArticlePubMedGoogle Scholar
- Sonenshine, D. E. & Stewart, P. E. Microbiomes of blood-feeding arthropods: genes coding for essential nutrients and relation to vector fitness and pathogenic infections. A review. Microorganisms9, 2433 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chabanol, E., Behrends, V., Prevot, G., Christophides, G. K. & Gendrin, M. Antibiotic treatment in Anopheles coluzzii affects carbon and nitrogen metabolism. Pathogens9, 679 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Guegan, M. et al. Mosquito sex and mycobiota contribute to fructose metabolism in the Asian tiger mosquito Aedes albopictus. Microbiome10, 138 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Guegan, M. et al. Who is eating fructose within the Aedes albopictus gut microbiota? Environ. Microbiol.22, 1193–1206 (2020). ArticleCASPubMedGoogle Scholar
- Minard, G. et al. Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol. Ecol.83, 63–73 (2013). ArticleCASPubMedGoogle Scholar
- Caragata, E. P., Rances, E., O’Neill, S. L. & McGraw, E. A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol.67, 205–218 (2014). ArticleCASPubMedGoogle Scholar
- Nascimento da Silva, J. et al. Wolbachia pipientis modulates metabolism and immunity during Aedes fluviatilis oogenesis. Insect Biochem. Mol. Biol.146, 103776 (2022). ArticleCASPubMedGoogle Scholar
- Didion, E. M. et al. Microbiome reduction prevents lipid accumulation during early diapause in the northern house mosquito, Culex pipiens pipiens. J. Insect Physiol.134, 104295 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Giraud, E. et al. Mosquito–bacteria interactions during larval development trigger metabolic changes with carry-over effects on adult fitness. Mol. Ecol.31, 1444–1460 (2022). ArticleCASPubMedGoogle Scholar
- Chen, S., Johnson, B. K., Yu, T., Nelson, B. N. & Walker, E. D. Elizabethkingia anophelis: physiologic and transcriptomic responses to iron stress. Front. Microbiol.11, 804 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Martin, E. et al. The mycobiota of the sand fly Phlebotomus perniciosus: involvement of yeast symbionts in uric acid metabolism. Environ. Microbiol.20, 1064–1077 (2018). ArticleCASPubMedGoogle Scholar
- Ganley, J. G. et al. A systematic analysis of mosquito–microbiome biosynthetic gene clusters reveals antimalarial siderophores that reduce mosquito reproduction capacity. Cell Chem. Biol.27, 817–826 (2020). ArticleCASPubMedGoogle Scholar
- Feng, Y. et al. Anopheline mosquitoes are protected against parasite infection by tryptophan catabolism in gut microbiota. Nat. Microbiol.7, 707–715 (2022). ArticleCASPubMedGoogle Scholar
- Molloy, J. C., Sommer, U., Viant, M. R. & Sinkins, S. P. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol.82, 3109–3120 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Manokaran, G. et al. Modulation of acyl-carnitines, the broad mechanism behind Wolbachia-mediated inhibition of medically important flaviviruses in Aedes aegypti. Proc. Natl Acad. Sci. USA117, 24475–24483 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Haqshenas, G. et al. A role for the insulin receptor in the suppression of Dengue virus and Zika virus in Wolbachia-infected mosquito cells. Cell Rep.26, 529–535 (2019). ArticleCASPubMedGoogle Scholar
- Rio, R. V. et al. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: glossinidae) obligate symbiont Wigglesworthia. mBio3, e00240-11 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Rio, R. V. M. et al. Mutualist-provisioned resources impact vector competency. mBio10, e00018–e00019 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, M. et al. Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH. Cell Rep.35, 108992 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kelly, P. H. et al. The gut microbiome of the vector Lutzomyia longipalpis is essential for survival of Leishmania infantum. mBio8, e01121-16 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Louradour, I. et al. The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cell Microbiol.19, 10.1111 (2017). ArticleGoogle Scholar
- Dickson, L. B. et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv.3, e1700585 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Matetovici, I., De Vooght, L. & Van Den Abbeele, J. Innate immunity in the tsetse fly (Glossina), vector of African trypanosomes. Dev. Comp. Immunol.98, 181–188 (2019). ArticleCASPubMedGoogle Scholar
- Salcedo-Porras, N. & Lowenberger, C. The innate immune system of kissing bugs, vectors of chagas disease. Dev. Comp. Immunol.98, 119–128 (2019). ArticleCASPubMedGoogle Scholar
- Serafim, T. D. et al. Leishmaniasis: the act of transmission. Trends Parasitol.37, 976–987 (2021). ArticleCASPubMedGoogle Scholar
- Fogaca, A. C. et al. Tick immune system: what is known, the interconnections, the gaps, and the challenges. Front. Immunol.12, 628054 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bartholomay, L. C. & Michel, K. Mosquito immunobiology: the intersection of vector health and vector competence. Annu. Rev. Entomol.63, 145–167 (2018). ArticleCASPubMedGoogle Scholar
- Weiss, B. L., Wang, J. & Aksoy, S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol.9, e1000619 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Weiss, B. L., Wang, J., Maltz, M. A., Wu, Y. & Aksoy, S. Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLoS Pathog.9, e1003318 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Weiss, B. L., Maltz, M. & Aksoy, S. Obligate symbionts activate immune system development in the tsetse fly. J. Immunol.188, 3395–3403 (2012). ArticleCASPubMedGoogle Scholar
- Trappeniers, K., Matetovici, I. & Van Den Abbeele, J. & De Vooght, L. The tsetse fly displays an attenuated immune response to its secondary symbiont, Sodalis glossinidius. Front. Microbiol.10, 1650 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Benoit, J. B. et al. Symbiont-induced odorant binding proteins mediate insect host hematopoiesis. eLife6, e19535 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Meister, S. et al. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog.5, e1000542 (2009). ArticlePubMedPubMed CentralGoogle Scholar
- Wang, J., Wu, Y., Yang, G. & Aksoy, S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc. Natl Acad. Sci. USA106, 12133–12138 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Salcedo-Porras, N., Noor, S., Cai, C., Oliveira, P. L. & Lowenberger, C. Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the IMD pathway. Curr. Res. Insect Sci.1, 100006 (2021). ArticleCASPubMedGoogle Scholar
- Rodgers, F. H. et al. Functional analysis of the three major PGRPLC isoforms in the midgut of the malaria mosquito Anopheles coluzzii. Insect Biochem. Mol. Biol.118, 103288 (2020). ArticleCASPubMedGoogle Scholar
- Gao, L., Song, X. & Wang, J. Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection. Parasit. Vectors13, 3 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Gendrin, M. et al. The peptidoglycan recognition proteins PGRPLA and PGRPLB regulate Anopheles immunity to bacteria and affect infection by Plasmodium. J. Innate Immun.9, 333–342 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Xiao, X. et al. A Mesh–Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol.2, 17020 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Oliveira, J. H. et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog.7, e1001320 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Diaz-Albiter, H., Sant’Anna, M. R., Genta, F. A. & Dillon, R. J. Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the sand phlebotomine fly Lutzomyia longipalpis. J. Biol. Chem.287, 23995–24003 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, J. & Aksoy, S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse’s offspring. Proc. Natl Acad. Sci. USA109, 10552–10557 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Song, X., Wang, M., Dong, L., Zhu, H. & Wang, J. PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis. PLoS Pathog.14, e1006899 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Pang, X. et al. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol.1, 16023 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pan, X. et al. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME J.12, 277–288 (2018). ArticleCASPubMedGoogle Scholar
- Rodrigues, J., Brayner, F. A., Alves, L. C., Dixit, R. & Barillas-Mury, C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science329, 1353–1355 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dey, R. et al. Gut microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1β. Cell Host Microbe23, 134–143 (2018). ArticleCASPubMedGoogle Scholar
- Wei, N., Cao, J., Zhang, H., Zhou, Y. & Zhou, J. The tick microbiota dysbiosis promote tick-borne pathogen transstadial transmission in a Babesia microti-infected mouse model. Front. Cell Infect. Microbiol.11, 713466 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Narasimhan, S. et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe15, 58–71 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Abraham, N. M. et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc. Natl Acad. Sci. USA114, E781–E790 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wu, P. et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe25, 101–112 (2019). ArticleCASPubMedGoogle Scholar
- Kumar, S., Molina-Cruz, A., Gupta, L., Rodrigues, J. & Barillas-Mury, C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science327, 1644–1648 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rodgers, F. H., Gendrin, M., Wyer, C. A. S. & Christophides, G. K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog.13, e1006391 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Narasimhan, S. et al. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun.8, 184 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Raddi, G. et al. Mosquito cellular immunity at single-cell resolution. Science369, 1128–1132 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ferreira Barletta, A. B. et al. Hemocyte differentiation to the megacyte lineage enhances mosquito immunity against Plasmodium. eLife11, e81116 (2022). ArticlePubMedGoogle Scholar
- Yan, Y. et al. The immune deficiency and c-Jun N-terminal kinase pathways drive the functional integration of the immune and circulatory systems of mosquitoes. Open Biol.12, 220111 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ramirez, J. L. et al. The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate Immun.6, 119–128 (2014). ArticleCASPubMedGoogle Scholar
- Olmo, R. P. et al. Mosquito vector competence for dengue is modulated by insect-specific viruses. Nat. Microbiol.8, 135–149 (2023). ArticleCASPubMedGoogle Scholar
- Gao, H. et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol.6, 806–817 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Valzano, M. et al. A yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malar. J.15, 21 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Yu, X. et al. Lipases secreted by a gut bacterium inhibit arbovirus transmission in mosquitoes. PLoS Pathog.18, e1010552 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Saraiva, R. G. et al. Chromobacterium spp. mediate their anti-Plasmodium activity through secretion of the histone deacetylase inhibitor romidepsin. Sci. Rep.8, 6176 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- da Mota, F. F. et al. In vitro trypanocidal activity, genomic analysis of isolates, and in vivo transcription of type VI secretion system of Serratia marcescens belonging to the microbiota of Rhodnius prolixus digestive tract. Front. Microbiol.9, 3205 (2018). ArticlePubMedGoogle Scholar
- Zimmer, K. R. et al. Cattle tick-associated bacteria exert anti-biofilm and anti-Tritrichomonas foetus activities. Vet. Microbiol.164, 171–176 (2013). ArticleCASPubMedGoogle Scholar
- Apte-Deshpande, A., Paingankar, M., Gokhale, M. D. & Deobagkar, D. N. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE7, e40401 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kaur, R. et al. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe29, 879–893 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Alam, U. et al. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog.7, e1002415 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bian, G. et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science340, 748–751 (2013). ArticleCASPubMedGoogle Scholar
- Durvasula, R. V. et al. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc. Natl Acad. Sci. USA94, 3274–3278 (1997). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, S. et al. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl Acad. Sci. USA109, 12734–12739 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Shane, J. L., Grogan, C. L., Cwalina, C. & Lampe, D. J. Blood meal-induced inhibition of vector-borne disease by transgenic microbiota. Nat. Commun.9, 4127 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- De Vooght, L., Caljon, G., De Ridder, K. & Van Den Abbeele, J. Delivery of a functional anti-trypanosome nanobody in different tsetse fly tissues via a bacterial symbiont, Sodalis glossinidius. Microb. Cell Fact.13, 156 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Yang, L. et al. Paratransgenic manipulation of a tsetse microRNA alters the physiological homeostasis of the fly’s midgut environment. PLoS Pathog.17, e1009475 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Taracena, M. L. et al. Genetically modifying the insect gut microbiota to control Chagas disease vectors through systemic RNAi. PLoS Negl. Trop. Dis.9, e0003358 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Ratcliffe, N. A. et al. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasit. Vectors15, 112 (2022). ArticlePubMedPubMed CentralGoogle Scholar
- Maitre, A. et al. Vector microbiota manipulation by host antibodies: the forgotten strategy to develop transmission-blocking vaccines. Parasit. Vectors15, 4 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ben-Yakir, D. Growth retardation of Rhodnius prolixus symbionts by immunizing host against Nocardia (Rhodococcus) rhodnii. J. Insect Physiol.33, 379–383 (1987). ArticleGoogle Scholar
- Mateos-Hernandez, L. et al. Anti-microbiota vaccines modulate the tick microbiome in a taxon-specific manner. Front. Immunol.12, 704621 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mateos-Hernandez, L. et al. Anti-tick microbiota vaccine impacts Ixodes ricinus performance during feeding. Vaccines8, 702 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Azelyte, J. et al. Anti-microbiota vaccine reduces avian malaria infection within mosquito vectors. Front. Immunol.13, 841835 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Noden, B. H., Vaughan, J. A., Pumpuni, C. B. & Beier, J. C. Mosquito ingestion of antibodies against mosquito midgut microbiota improves conversion of ookinetes to oocysts for Plasmodium falciparum, but not P. yoelii. Parasitol. Int.60, 440–446 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Frankel-Bricker, J., Buerki, S., Feris, K. P. & White, M. M. Influences of a prolific gut fungus (Zancudomyces culisetae) on larval and adult mosquito (Aedes aegypti)-associated microbiota. Appl. Environ. Microbiol.86, e02334-19 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Bai, L., Wang, L., Vega-Rodriguez, J., Wang, G. & Wang, S. A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front. Microbiol.10, 1580 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Cappelli, A. et al. Asaia activates immune genes in mosquito eliciting an anti-Plasmodium response: implications in malaria control. Front. Genet.10, 836 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Saraiva, R. G. et al. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl. Trop. Dis.12, e0006443 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Ramirez, J. L. et al. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog.10, e1004398 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Moreira, L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell139, 1268–1278 (2009). ArticlePubMedGoogle Scholar
- Blandin, S. A., Marois, E. & Levashina, E. A. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe3, 364–374 (2008). ArticleCASPubMedGoogle Scholar
- Tikhe, C. V. & Dimopoulos, G. Mosquito antiviral immune pathways. Dev. Comp. Immunol.116, 103964 (2021). ArticleCASPubMedGoogle Scholar
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (U1902211) and Science and Technology Leading Team from Inner Mongolia, China (2022SLJRC0023) to J.W.








